The standard treatment of health is with health Authorities approved medicine, so-called Allopathic medicine being another term for conventional, or modern Western medicine. It is an evidence-based system where doctors and other healthcare professionals treat symptoms using conventional medications. Allopathic medicines rely on the supposition that single organic compounds can enter into the complex human organic system and alter some activity “pathway” which is designed to interrupt the progress of a disease condition or even eliminate it. Allopathic medicine targets the symptoms at a specific organ or part that is affected in the body, but there is always a risk of side effects and infection spreading to the neighboring body parts. Patients, but also doctors, however, prefer Allopathic medicine because of it is convenient to people´s hectic Lifestyle, being easily available, at your next door pharmacy.
In the end of the 20th Century science developed various alternative treatment methods like new regenerative cellular medicine and Energy related methods, to improve health and quality of life, which however, is still under review by regulatory authorities to evaluate its safety in human beings. With the increase of geriatric syndromes and rare incurable diseases due to aging, the demand for regenerative medicine (RM) is increasing (Cossu et al., 2018). Geriatric syndromes include a number of conditions typical of, if not specific to, aging, such as dementia, depression, delirium, incontinence, vertigo, falls, spontaneous bone fractures, failure to thrive, and neglect and abuse. Geriatric syndromes are associated with reduced life expectancy and reduced quality of life with aging. Regenerative Medicine (RM) or cell therapy products (CTP) have the potential to repair or reconstruct damaged functional cells and tissues for unmet medical needs such as dementia and organ defects (Witten et al., 2015).
Under the umbrella of Regenerative Medicine, Stem cell therapy has developed in recent years to become an alternative and supporting tool to treat unmet medical needs. Today we can divide stem cells in four main categories.
1. Embryonic stem cells, (ESCs) are found in the inner cell mass of the human blastocyst, an early stage of the developing
embryo lasting from the 4th to 7th day after fertilization. In normal embryonic development, they disappear after the 7th day,
and begin to form the three embryonic tissue layers, consisting of the endoderm (inner layer), the ectoderm (outer layer), and
the mesoderm (middle layer).
2. Haematopoietic Stem cells (HSCs) are multipotent primitive cells that can develop into all types of blood cells, incl. myeloid-
lineage and lymphoid-lineage cells. HSCs can be found in several organs, such as peripheral blood (PB), bone marrow (BM),
and umbilical cord blood (UCB).
3. Mesenchymal Stem Cells (MSCs) have been shown to be perivascular in vivo, the existing traditional view that focuses on the
multipotent differentiation capacity of these cells should be expanded to include their equally interesting role as cellular
modulators that brings them into a broader therapeutic scenario. We discuss existing evidence that leads us to propose that
during local injury, MSCs are released from their perivascular location, become activated and establish a regenerative
microenvironment by secreting bioactive molecules and regulating the local immune response. These trophic and
immunomodulatory activities suggest that MSCs may serve as site-regulated “drugstores” in vivo.
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3144500/ ARNOLD I. CAPLAN, Ph.D. and DIEGO CORREA, M.D., Ph.D.
4. Induced Pluripotent Stem Cells (iPS), are a type of pluripotent stem cell derived from adult somatic cells that have been
genetically reprogrammed to an embryonic stem (ES) cell-like state through the forced expression of genes and factors
important for maintaining the defining properties of ES cells.
Mesenchymal Stem Cells are considered the natural repair team of the human cellular structure and has gained enormous international interest in improving health of patients, even are considered to support longevity by adding new stem cells into a human body to reverse the natural stem cell exhaustion a body is facing with aging. We are focusing our activities on Mesenchymal stem cells from Cord tissue / Wharton Jelly.
Although science has shown that Mesenchymal Stem Cells (MSCs) are safe to use in humans regulatory agencies in many countries are still reviewing it's safety in various applications. MSCs are naturally available in a human body and should not be regulated as a drug which in turn could be patented by the industry, although FDA but also EMA disputing this, in spite of the fact that the Court in California in December, 2022, sided with Cell Surgical Network, allowing them to use autologous stem cells for various conditions in IRB approved protocols, i.e. FDA is not regulating such medical procedures with natural available stem cells!
Therefore, before engaging in stem cell therapies it is necessary to review the legality in those countries offering stem cell therapies. Let me explain the legal framework which permits to provide stem cell therapies.
1. Approved drug by FDA, EMA or other regulatory agencies in any Country.
2. Participating in approved clinical trials
3. Providing autologous stem cells from adipose tissues or bone marrow as a medical procedure with IRB approved
protocols in countries where this is legalised.
4. During the Islamic World League meeting held in Makkah al-Mukarramah, Saudi Arabia, in 2003, the Islamic Fiqh Council
has agreed that it is permitted in Muslim countries to obtain, grow, and use stem cells for therapeutic or permissible
scientific research, if the source of the specimen is legitimate, i.e. not embryonic! (Muslim World League, 2020).
5. European specific Hospital Exemption in EU member countries. mainly provided legally in Germany, Poland, Slovakia but
also The Netherlands and Lithuania.
6. Compassionate Use; as per EU Regulation 726/2004, Article 831, Compassionate Use means to make unapproved
medicinal products available to a group of patients that are suffering from a life-threatening, chronically or seriously
debilitating disease, that otherwise cannot be adequately treated.
The legal basis for Compassionate Use is an EU regulation, and accordingly, the expectation should be that this is directly
legally applicable in each member state. Surprisingly, beyond the legal basis in 726/2004, the regulations in each member
state have little in common. Actually, those national legal requirements show an immense variety and even
contradicting qualities.
As an Example: The Austrian Medicinal Products Act (Arzneimittelgesetz, AMG), as outlined in § 8 para. 1 Z 2 AMG
permits the treatment as " individueler Heilversuch /named patient use" of patients with medicinal products that are
not licensed in Austria when specific requirements are met:
§ 8. (1) Medicinal products do not require a license, if...
a medical doctor, dentist or veterinarian, licensed for independent exercise of profession in Austria, confirms, that the
medicinal product is urgently required to avert a life threatening situation or severe health damage, and that
successful treatment likely cannot be achieved with a licensed and available medicinal product according to the
scientific state of the art.
In contrast to compassionate use programs that apply to a group/cohort of patients, named patient use, as the name
implies, refers to a single individual. Named patient use in Austria does not require notification to or approval by the
Austrian Federal Office for Safety in Health Care and lies in the sole responsibility of the treating physician.
UK: According to EMA´s report the UK, before Brexit, has granted 18 authorisations to manufacture and supply
unlicensed ATMPs under the terms of the exemption provided by Article 5(1) of Directive 2001/83/EC (the UK’s Specials
scheme) have been granted. The UK has developed post Brexit guidance for arrangements under the hospital exemption
scheme.
Additional cellular therapy for Cancer:
7. A Lithuanian clinic is providing legalized Dendritic cell therapy to increase the natural capacity of the immune system
to fight cancer cells.
We have carefully screened the various legal possibilities and are associated in the USA with Stem Surgical Network for MSCs from adipose tissues and in Europe with Innovita in Lithuania providing Stem Cell and Dendritic cell therapy in a hospital with a hospital exemption registration using MSCs and Dendritic Cells registered as ATMP.
In the end of the 20th Century science developed various alternative treatment methods like new regenerative cellular medicine and Energy related methods, to improve health and quality of life, which however, is still under review by regulatory authorities to evaluate its safety in human beings. With the increase of geriatric syndromes and rare incurable diseases due to aging, the demand for regenerative medicine (RM) is increasing (Cossu et al., 2018). Geriatric syndromes include a number of conditions typical of, if not specific to, aging, such as dementia, depression, delirium, incontinence, vertigo, falls, spontaneous bone fractures, failure to thrive, and neglect and abuse. Geriatric syndromes are associated with reduced life expectancy and reduced quality of life with aging. Regenerative Medicine (RM) or cell therapy products (CTP) have the potential to repair or reconstruct damaged functional cells and tissues for unmet medical needs such as dementia and organ defects (Witten et al., 2015).
Under the umbrella of Regenerative Medicine, Stem cell therapy has developed in recent years to become an alternative and supporting tool to treat unmet medical needs. Today we can divide stem cells in four main categories.
1. Embryonic stem cells, (ESCs) are found in the inner cell mass of the human blastocyst, an early stage of the developing
embryo lasting from the 4th to 7th day after fertilization. In normal embryonic development, they disappear after the 7th day,
and begin to form the three embryonic tissue layers, consisting of the endoderm (inner layer), the ectoderm (outer layer), and
the mesoderm (middle layer).
2. Haematopoietic Stem cells (HSCs) are multipotent primitive cells that can develop into all types of blood cells, incl. myeloid-
lineage and lymphoid-lineage cells. HSCs can be found in several organs, such as peripheral blood (PB), bone marrow (BM),
and umbilical cord blood (UCB).
3. Mesenchymal Stem Cells (MSCs) have been shown to be perivascular in vivo, the existing traditional view that focuses on the
multipotent differentiation capacity of these cells should be expanded to include their equally interesting role as cellular
modulators that brings them into a broader therapeutic scenario. We discuss existing evidence that leads us to propose that
during local injury, MSCs are released from their perivascular location, become activated and establish a regenerative
microenvironment by secreting bioactive molecules and regulating the local immune response. These trophic and
immunomodulatory activities suggest that MSCs may serve as site-regulated “drugstores” in vivo.
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3144500/ ARNOLD I. CAPLAN, Ph.D. and DIEGO CORREA, M.D., Ph.D.
4. Induced Pluripotent Stem Cells (iPS), are a type of pluripotent stem cell derived from adult somatic cells that have been
genetically reprogrammed to an embryonic stem (ES) cell-like state through the forced expression of genes and factors
important for maintaining the defining properties of ES cells.
Mesenchymal Stem Cells are considered the natural repair team of the human cellular structure and has gained enormous international interest in improving health of patients, even are considered to support longevity by adding new stem cells into a human body to reverse the natural stem cell exhaustion a body is facing with aging. We are focusing our activities on Mesenchymal stem cells from Cord tissue / Wharton Jelly.
Although science has shown that Mesenchymal Stem Cells (MSCs) are safe to use in humans regulatory agencies in many countries are still reviewing it's safety in various applications. MSCs are naturally available in a human body and should not be regulated as a drug which in turn could be patented by the industry, although FDA but also EMA disputing this, in spite of the fact that the Court in California in December, 2022, sided with Cell Surgical Network, allowing them to use autologous stem cells for various conditions in IRB approved protocols, i.e. FDA is not regulating such medical procedures with natural available stem cells!
Therefore, before engaging in stem cell therapies it is necessary to review the legality in those countries offering stem cell therapies. Let me explain the legal framework which permits to provide stem cell therapies.
1. Approved drug by FDA, EMA or other regulatory agencies in any Country.
2. Participating in approved clinical trials
3. Providing autologous stem cells from adipose tissues or bone marrow as a medical procedure with IRB approved
protocols in countries where this is legalised.
4. During the Islamic World League meeting held in Makkah al-Mukarramah, Saudi Arabia, in 2003, the Islamic Fiqh Council
has agreed that it is permitted in Muslim countries to obtain, grow, and use stem cells for therapeutic or permissible
scientific research, if the source of the specimen is legitimate, i.e. not embryonic! (Muslim World League, 2020).
5. European specific Hospital Exemption in EU member countries. mainly provided legally in Germany, Poland, Slovakia but
also The Netherlands and Lithuania.
6. Compassionate Use; as per EU Regulation 726/2004, Article 831, Compassionate Use means to make unapproved
medicinal products available to a group of patients that are suffering from a life-threatening, chronically or seriously
debilitating disease, that otherwise cannot be adequately treated.
The legal basis for Compassionate Use is an EU regulation, and accordingly, the expectation should be that this is directly
legally applicable in each member state. Surprisingly, beyond the legal basis in 726/2004, the regulations in each member
state have little in common. Actually, those national legal requirements show an immense variety and even
contradicting qualities.
As an Example: The Austrian Medicinal Products Act (Arzneimittelgesetz, AMG), as outlined in § 8 para. 1 Z 2 AMG
permits the treatment as " individueler Heilversuch /named patient use" of patients with medicinal products that are
not licensed in Austria when specific requirements are met:
§ 8. (1) Medicinal products do not require a license, if...
a medical doctor, dentist or veterinarian, licensed for independent exercise of profession in Austria, confirms, that the
medicinal product is urgently required to avert a life threatening situation or severe health damage, and that
successful treatment likely cannot be achieved with a licensed and available medicinal product according to the
scientific state of the art.
In contrast to compassionate use programs that apply to a group/cohort of patients, named patient use, as the name
implies, refers to a single individual. Named patient use in Austria does not require notification to or approval by the
Austrian Federal Office for Safety in Health Care and lies in the sole responsibility of the treating physician.
UK: According to EMA´s report the UK, before Brexit, has granted 18 authorisations to manufacture and supply
unlicensed ATMPs under the terms of the exemption provided by Article 5(1) of Directive 2001/83/EC (the UK’s Specials
scheme) have been granted. The UK has developed post Brexit guidance for arrangements under the hospital exemption
scheme.
Additional cellular therapy for Cancer:
7. A Lithuanian clinic is providing legalized Dendritic cell therapy to increase the natural capacity of the immune system
to fight cancer cells.
We have carefully screened the various legal possibilities and are associated in the USA with Stem Surgical Network for MSCs from adipose tissues and in Europe with Innovita in Lithuania providing Stem Cell and Dendritic cell therapy in a hospital with a hospital exemption registration using MSCs and Dendritic Cells registered as ATMP.
Therefore we are unique in providing in Europe, in a legalized hospital, various stem cell therapies
If you are interested in a European Stem Cell or Dendritic cell therapy, please kindly contact us with your medical history and a present medical assessment including medication used to review whether you are eligible for a cellular therapy. After the review, we will invite you to a Zoom video conference to discuss our findings with the Medical doctor of our associated hospital in Lithuania. Once we and you agree on the assessment, the potential therapy and its costs, the performing doctor will provide a prescription with details of assessment and the products used in the patient funded medical procedure. We will then confirm the date of the treatment, support the booking of a hotel, arrange the pick up from the hotel to the hospital and the return to the hotel, as well as supporting flights from your airport to the airport in Lithuania as well as all its pick ups by taxis.
We are certain you will enjoy the historic country of Lithuania as well as the treatment in a legalized environment. Do not hesitate to contact us for a free of charge review (Zoom Conference) of your case.
Our partner is studying the investigational use with allogeneic Mesenchymal Stem cells from Wharton Jelly and Dendritic cell therapy approved as ATMP in a hospital with a legalized hospital exemption registration for the following conditions:
Treatment prices will depend on condition and assessments of the patient's medical history and its actual documentation, but in general include fully licensed Physician costs, local blood work tests and analysis, radiology and imaging costs if needed and cellular therapy via IV drips as prescribed. Depending on the health assessment, treatment costs are varying from 18.000 Euros to 25.000 Euros plus. Flights and transportation, hotel accommodation, meals and entertainment is not included in our prices but Aristoloft and it´s local partners are providing appropriate support for it.
In case of need a member of Aristoloft will accompany the patient for a standard fee of 1.800 Euros to support the patient´s stay and treatment arrangements onsite. Depending on the condition treated a patient will need to stay between 4 to 6 days in the hotel to accompany the treatment in the hospital.
- ATMP Stem Cell Therapy for Autoimmune Disease and Chronic Degenerative Treatments
- ATMP Stem Cell Therapy for Orthopedics
- ATMP Exosomes Treatments
- Anti-Aging Longevity Treatments
- ATMP Cell Immunotherapy for Cancer
Treatment prices will depend on condition and assessments of the patient's medical history and its actual documentation, but in general include fully licensed Physician costs, local blood work tests and analysis, radiology and imaging costs if needed and cellular therapy via IV drips as prescribed. Depending on the health assessment, treatment costs are varying from 18.000 Euros to 25.000 Euros plus. Flights and transportation, hotel accommodation, meals and entertainment is not included in our prices but Aristoloft and it´s local partners are providing appropriate support for it.
In case of need a member of Aristoloft will accompany the patient for a standard fee of 1.800 Euros to support the patient´s stay and treatment arrangements onsite. Depending on the condition treated a patient will need to stay between 4 to 6 days in the hotel to accompany the treatment in the hospital.
If you are interested in a European Stem Cell or Dendritic cell therapy, please kindly contact us with your medical history and a present medical assessment including medication used to review whether you are eligible for a cellular therapy.
As mentioned before stem cell therapy is very "controversial, and rather a political issue" when it comes to health regulations in the various countries. Our science based information and review of legal possibilities in a given country is independent from any political interpretation and based on local confirmed regulations.
Stem cells are the building block of a human cellular structure which is regenerating itself via stem cells from birth to end of life. During lifetime stem cells are used up for its repair and maintenance activity in the body and as such over time the quantity and quality of stem cells for this natural healing process is fading away. Easily to see when a young person cuts its finger, it heals very quickly whereas in an elderly person the process of healing takes longer and often shows scaring. This situation is called stem cell exhaustion.
Many clinics worldwide are providing stem cell therapies to the displeasure of regulatory agencies and the industry because, instead of using expensive symptom relieving drugs, approved by the mono molecular drug´s mode of action and its clinical pathway reflecting the safety and effectivity demands of heavy manipulated drugs, stem cell clinics, are using the natural healing power of stem cells with a multi molecular mode of action.
Science has shown that stem cells are a very intelligent multi molecular structure, who, with it´s natural embedded Know-how, expressed via its homing mechanism, knows exactly when, where and what to repair often even curing the root cause of a disease.
Under FDA regulations, an Institutional Review Board (IRB), is a group of professionals that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
Medical procedures are regulated by IRBs, not regulated in the USA by the FDA. The only purpose of reviews of the Institutional Reviewing Board (IRB) by the FDA is to assure, both in advance and by periodic review, that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in the research. To accomplish this purpose, IRBs use a group process to review research protocols and related materials, e.g., informed consent documents and investigator brochures to ensure protection of the rights and welfare of human subjects of research.
We have to point out, that globally, a medical procedure based on an IRB protocol are not regulated by drug approving authorities, like the FDA or EMA, although those organisations try with guidelines to impose their influence on health related matters.
We furthermore like to emphasize that the need and its form of FDA regulations are based on the early 20th century philosophy and introduction of industrialized produced drugs, which did encounter problems in quality and safety in this early years of drug development. The production and science know how as well as its quality control and its application in the 21st Century is quite different and regulations should adopt to this change of knowledge, which clearly has been done already by various countries.
As mentioned before, science has advanced in the last 20 or so years exponentially, resulting in new and better understanding of the cellular structure of a body and its intervention. Furthermore autologous or even allogeneic stem cells used in medical procedures at clinics are not highly manipulated like chemical and biochemical drugs, or induced pluripotent stem cells highly modified from skin cells (so-called iPSC). When searching the internet you will finds many studies confirming the safety of stem cells and its exploratory therapies as a medical procedure, as well as early very positive results from FDA approved clinical trials, which regulatory authorities like to deney, stipulating them as too early and not yet fully documented results.
Besides the fact that thousands of clinics worldwide are doing stem cell therapies as a medical procedure very successful to improve quality of life in individual cases, the FDA lost its legal case against the Cell Surgical Network (CSN), California, last December (2022), as the US court ruled that stem cells from fat extracted by the CSN method and used at the same body are not drugs and therefore such a procedure is not regulated by the FDA.
Stem cells are the building block of a human cellular structure which is regenerating itself via stem cells from birth to end of life. During lifetime stem cells are used up for its repair and maintenance activity in the body and as such over time the quantity and quality of stem cells for this natural healing process is fading away. Easily to see when a young person cuts its finger, it heals very quickly whereas in an elderly person the process of healing takes longer and often shows scaring. This situation is called stem cell exhaustion.
Many clinics worldwide are providing stem cell therapies to the displeasure of regulatory agencies and the industry because, instead of using expensive symptom relieving drugs, approved by the mono molecular drug´s mode of action and its clinical pathway reflecting the safety and effectivity demands of heavy manipulated drugs, stem cell clinics, are using the natural healing power of stem cells with a multi molecular mode of action.
Science has shown that stem cells are a very intelligent multi molecular structure, who, with it´s natural embedded Know-how, expressed via its homing mechanism, knows exactly when, where and what to repair often even curing the root cause of a disease.
Under FDA regulations, an Institutional Review Board (IRB), is a group of professionals that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
Medical procedures are regulated by IRBs, not regulated in the USA by the FDA. The only purpose of reviews of the Institutional Reviewing Board (IRB) by the FDA is to assure, both in advance and by periodic review, that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in the research. To accomplish this purpose, IRBs use a group process to review research protocols and related materials, e.g., informed consent documents and investigator brochures to ensure protection of the rights and welfare of human subjects of research.
We have to point out, that globally, a medical procedure based on an IRB protocol are not regulated by drug approving authorities, like the FDA or EMA, although those organisations try with guidelines to impose their influence on health related matters.
We furthermore like to emphasize that the need and its form of FDA regulations are based on the early 20th century philosophy and introduction of industrialized produced drugs, which did encounter problems in quality and safety in this early years of drug development. The production and science know how as well as its quality control and its application in the 21st Century is quite different and regulations should adopt to this change of knowledge, which clearly has been done already by various countries.
As mentioned before, science has advanced in the last 20 or so years exponentially, resulting in new and better understanding of the cellular structure of a body and its intervention. Furthermore autologous or even allogeneic stem cells used in medical procedures at clinics are not highly manipulated like chemical and biochemical drugs, or induced pluripotent stem cells highly modified from skin cells (so-called iPSC). When searching the internet you will finds many studies confirming the safety of stem cells and its exploratory therapies as a medical procedure, as well as early very positive results from FDA approved clinical trials, which regulatory authorities like to deney, stipulating them as too early and not yet fully documented results.
Besides the fact that thousands of clinics worldwide are doing stem cell therapies as a medical procedure very successful to improve quality of life in individual cases, the FDA lost its legal case against the Cell Surgical Network (CSN), California, last December (2022), as the US court ruled that stem cells from fat extracted by the CSN method and used at the same body are not drugs and therefore such a procedure is not regulated by the FDA.
The general assumption is, that stem cell therapies as a medical procedure are safe, I personally did IV-injection of MSCs from umbilical cord tissues about 4 years ago the first time and have the energy and health of a much younger person at a 77 young cellular structure!
In order not to run into regulatory difficulties we have to point out that this website and its content cannot be considered as a medical advice, but reflects science opinion for educational purpose only.
Here are details of the activities of my US partner, Cell Surgical Network, who are studying the investigational use of Adipose Derived Stem Cells (ADSC's) for clinical research and deployment. We are inviting interested patients in the USA to review the site as well as contacting us for further questions if needed about such cellular therapies.
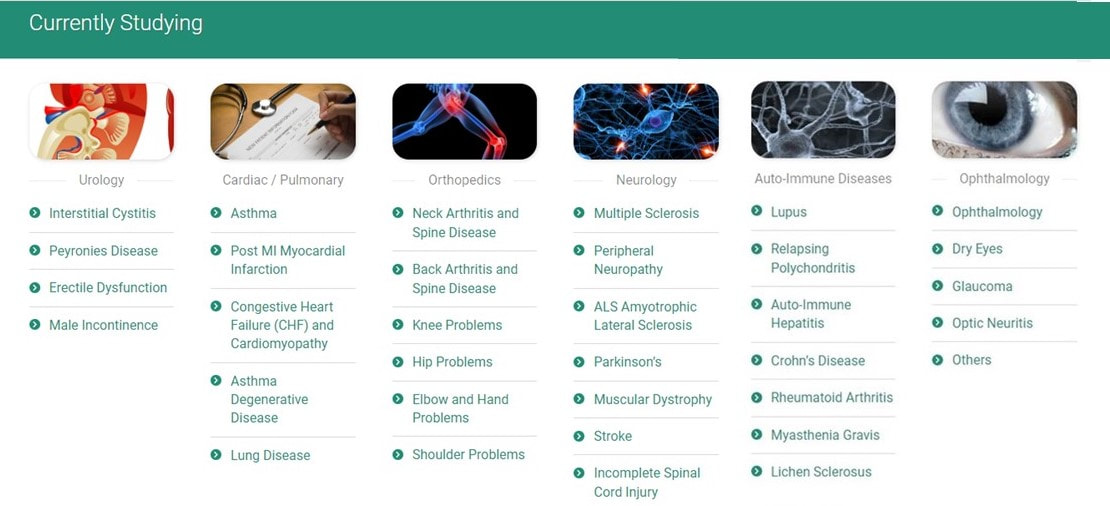
CSN is presently studying the following diseases with a patient's own stem cells, by harvesting fat cells in an about 20 minutes lasting specialist procedure from a person. After harvesting the fat, the stem cells are isolated from the fat and can be deployed through veins, arteries, spinal fluid, subcutaneously, or directly into joints or organs. The whole procedure is minimally invasive and lasts about three hours. As mentioned before if you are interest in any of the below investigating studies, please kindly contact us.
Aristoloft has various international partners and is studying stem cells in antiaging therapies also in Europe and India as well as Autism in a specialist European clinic. If you are interested in such therapies please do not hesitate to contact us.